- Overview
- Recommended Products
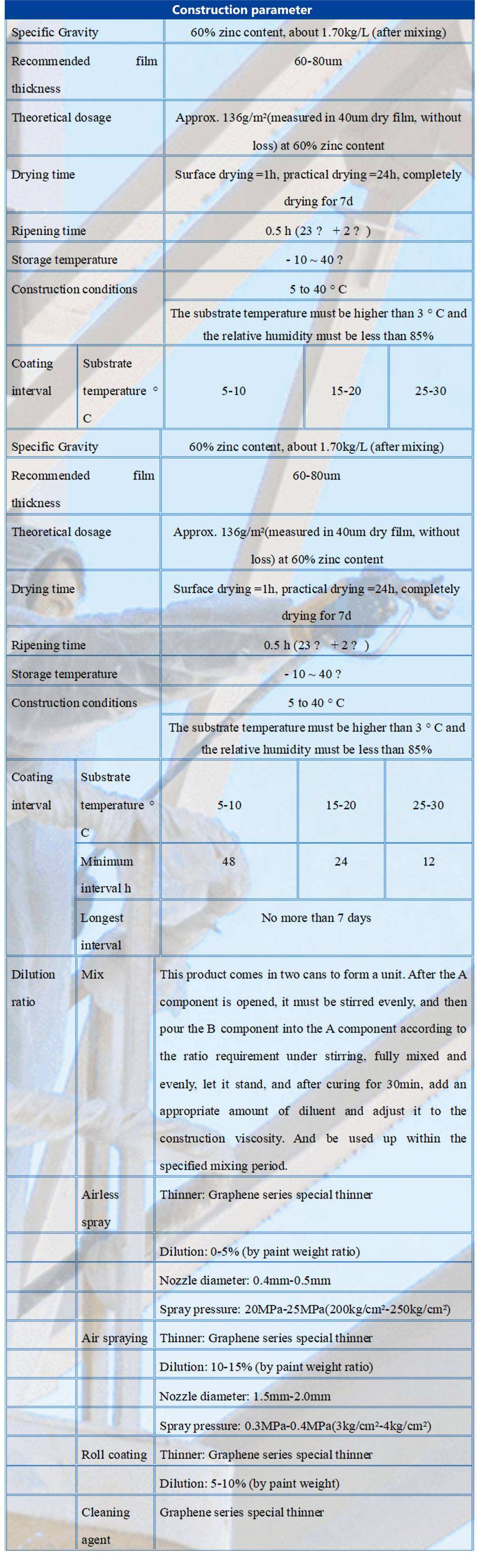
- Color Gray
- Proportioning main agent: Curing agent =25:3
- Construction brush coating, spraying, rolling coating can be
- The composition is composed of resin, zinc powder, graphene as the main raw materials, thickening agent, filler, auxiliary agent, solvent and curing agent.
- Excellent anti-corrosion properties. Graphene zinc powder primer using graphene sheet layer structure, can form a dense physical insulation layer, significantly improve the corrosion resistance of the product. Graphene's high specific surface area, excellent electrical conductivity, strength, toughness and shielding properties make it excellent in the field of anti-corrosion coatings.
- Improve the utilization of zinc powder. The traditional epoxy zinc-rich primer has some problems, such as low zinc powder utilization rate, high addition amount and easy cracking of thick coating. By adding graphene, the graphene zinc powder primer can make use of the conductive properties of graphene to form a conductive network at a lower zinc powder content, thereby improving the utilization rate of zinc powder and enhancing the overall anticorrosion ability of the coating.
- Salt spray resistance is improved. In the graphene zinc powder primer, the shielding effect of graphene can delay the penetration of corrosive media and reduce the electrolyte concentration in the coating, thus delaying the electrochemical corrosion consumption of metal zinc. This allows the metal zinc in the coating to play a cathodic protection role for a long time, and significantly improves the salt spray resistance of the coating.
- Environmental performance. Because the amount of zinc powder in the graphene zinc powder primer is reduced, the zinc oxide mist generated during the welding process is also reduced, reducing the impact on the environment and making it more environmentally friendly.
- Excellent comprehensive performance. Graphene zinc powder primer not only has the cathodic protection effect of epoxy zinc-rich coating and the shielding effect of glass flake coating, but also has good toughness, adhesion, water resistance and hardness. These comprehensive properties make the graphene zinc powder primer have wider applicability and higher cost performance while ensuring the anti-corrosion effect.
- Thermal stability and chemical stability. Graphene has excellent thermal and chemical stability and is able to remain stable under high temperature conditions as well as in corrosive or oxidizing environments, further enhancing the durability and reliability of the coating.
- Marine engineering: The anticorrosion property of graphene zinc powder primer is better than the existing epoxy zinc-rich coating, and can be widely used in Marine engineering coating protection.
- Transportation: Graphene zinc powder primer can be used for coating protection of transportation vehicles, such as ships, Bridges and so on.
- Large industrial equipment: graphene zinc powder primer can be used for painting protection of large industrial equipment, such as oil storage tanks, chemical equipment and so on.
- Special engineering facilities: graphene zinc powder primer can be used for painting protection of special engineering facilities, such as nuclear power plants, airport facilities, etc.
- Graphene zinc-rich primer + epoxy cloud iron intermediate paint/epoxy thick paste intermediate paint + acrylic polyurethane topcoat/polyurethane topcoat/polysiloxane topcoat/fluorocarbon topcoat/epoxy topcoat/alkyd topcoat/graphene topcoat/chlorinated rubber topcoat etc
- This product is like most zinc-rich paints, long-term exposure to the paint film will appear zinc salt, must be thoroughly cleaned before applying the next paint, otherwise it will affect the adhesion between layers.
- The temperature of the substrate must be above 3 ° C above the dew point, and when the temperature of the substrate is below 5 ° C, the paint film is not cured and should not be constructed.
- In the high temperature season construction, easy to occur dry spray, in order to avoid dry spray can be adjusted to dry spray until the diluent.
- This product should be used by professional painting operators according to the product packaging or the instructions in this manual.
- It is necessary to thoroughly remove oil and rust, etc., to achieve the rust removal standard Sa2.5, and the roughness reaches 30um-75um; Adopt manual rust removal method, need to reach the rust removal standard St3 level.
- Concrete surface should be flat, dry, no seepage floating and water. The base that has been polluted by grease and chemicals can be washed with detergent, lye or solvent, and can also be treated by fire baking, steam blowing, etc., but it must not damage the base.
- Products should be stored in a cool and ventilated place to prevent rain, direct sunlight, avoid collision, need to isolate the fire source.
- The construction site is strictly prohibited fireworks, painters should wear glasses, gloves, masks, etc., to avoid skin contact and inhalation of paint mist.
- All work of coating and use of this product must be carried out in accordance with various relevant national health, safety and environmental protection regulations and standards.
- If you have any questions about the use of this product, please contact our technical service department.
Graphene (Graphene) is a new material in which carbon atoms connected in a sp² hybrid are tightly packed into a single two-dimensional honeycomb lattice structure. Graphene has excellent optical, electrical and mechanical properties, and has important application prospects in materials science, micro and nano processing, energy, biomedicine and drug delivery, and is considered to be a revolutionary material in the future.
Andre Geim and Konstantin Novoselov, physicists at the University of Manchester in the United Kingdom, were awarded the 2010 Nobel Prize in Physics for successfully separating graphene from graphite by micromechanical stripping. The common powder production methods of graphene are mechanical stripping, REDOX method, SiC epitaxial growth method, and chemical vapor deposition (CVD) production method.
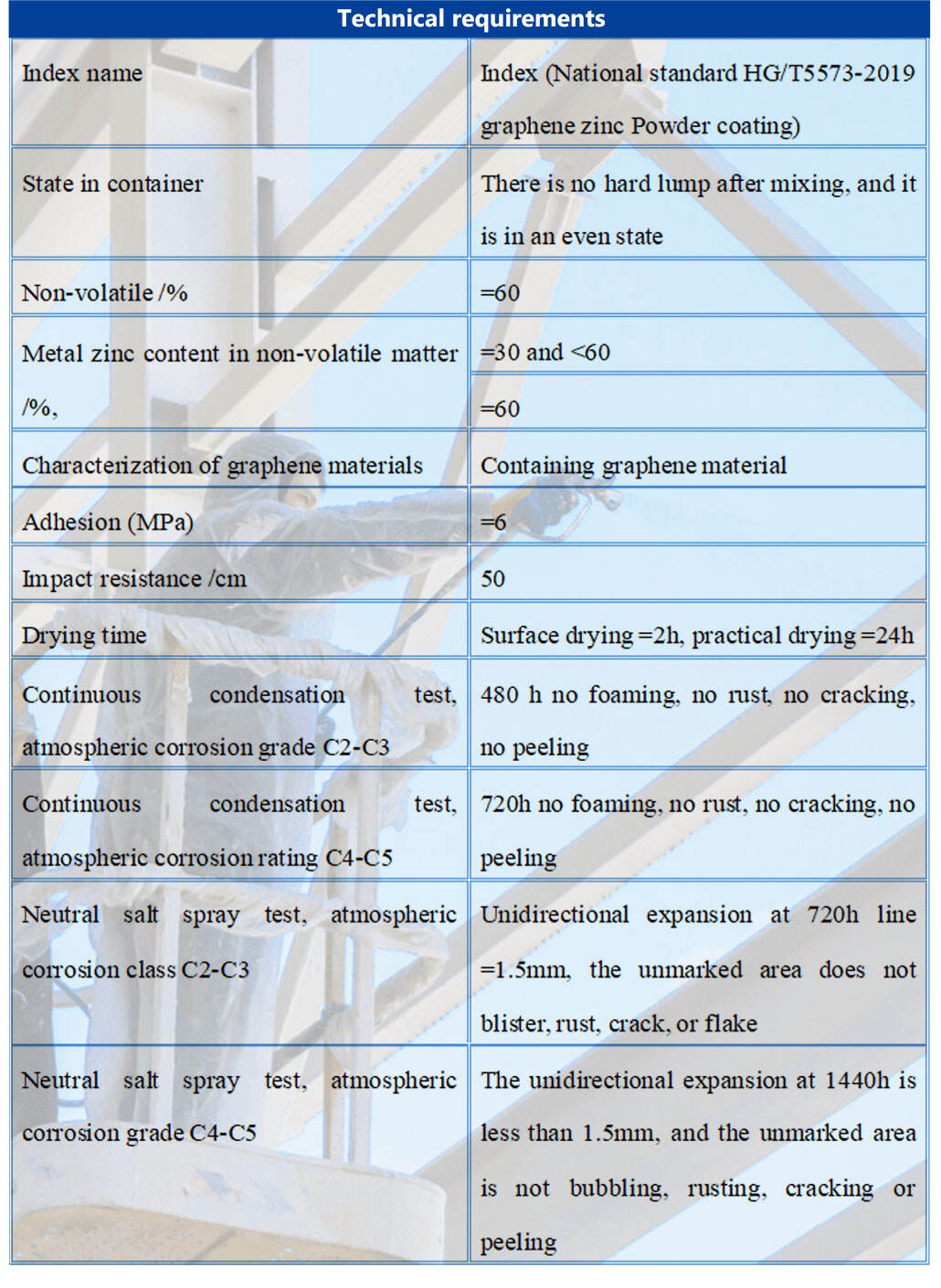
Basic parameters
Product characteristics
Product features
Product use

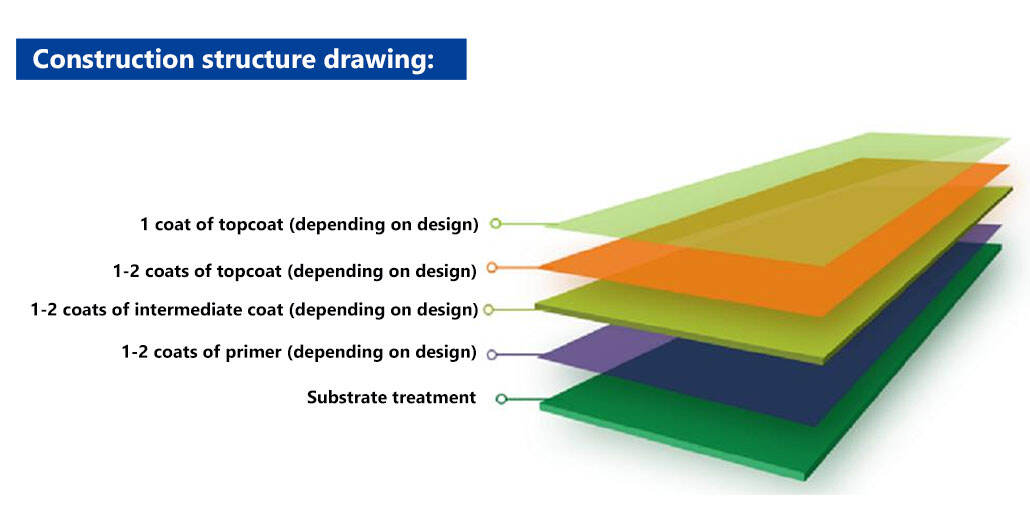
Supporting scheme:
Construction notes:
Steel surface:
Concrete surface:
Precautions
Supplement: About Graphene - Physicochemical Properties
I. Physical Properties:
Conductive and optical properties |
The arrangement of the carbon atoms in graphene is the same as that of the graphite monatomic layer to bond with sp hybrid orbitals, and has the following characteristics: carbon atoms have 4 valence electrons, of which 3 electrons generate sp bond, that is, each carbon atom contributes a non-bonded electron located in the pz orbital, and the pz orbital of the neighboring atom is perpendicular to the plane to form π bond. The newly formed π bond is in a half-filled state. The study confirmed that the carbon atoms in graphene have a coordination number of 3, the bond length between every two adjacent carbon atoms is 1.42×10 meters, and the Angle between the bonds is 120°. In addition to the cellular layered structure in which σ bonds are linked to other carbon atoms into hexagonal rings, the pz orbitals perpendicular to the layer plane of each carbon atom can form large π bonds (similar to the benzene ring) that run through the whole layer of multiple atoms, and thus have excellent electrical and optical properties. |
Mechanical properties |
Graphene is one of the strongest materials known, but it is also very ductile and can bend. Graphene has a theoretical Young's modulus of 1.0TPa and an inherent tensile strength of 130GPa. Reduced graphene modified by hydrogen plasma also has very good strength, with an average modulus of 0.25TPa greater. The graphite paper composed of graphene sheets has many holes, so the graphite paper is very brittle. However, the oxidized functional graphene is made into graphite paper from the functional graphene, which is extremely strong and strong. |
Electronic Effects |
The carrier mobility of graphene at room temperature is about 15,000 cm/(V·s), which is more than 10 times that of silicon materials and more than twice that of indium antimonide (InSb), the substance with the highest known carrier mobility. Under certain conditions such as low temperatures, the carrier mobility of graphene can even be as high as 250000cm/(V·s). Unlike many materials, graphene's electron mobility is less affected by temperature changes, and at any temperature between 50 and 500K, the electron mobility of a single layer of graphene is around 15,000 cm/(V·s). |
Thermal Properties |
Graphene has very good heat conduction properties. Pure, defect-free monolayer graphene has the highest thermal conductivity of any carbon material by far, with a thermal conductivity of up to 5300W/mK, higher than single-walled carbon nanotubes (3500W/mK) and multi-walled carbon nanotubes (3000W/mK). When it is used as a carrier, the thermal conductivity can also reach 600W/mK. In addition, the ballistic thermal conductivity of graphene can move down the lower limit of the ballistic thermal conductivity of carbon nanotubes per unit circumference and length. |
Optical Properties |
Graphene has very good optical properties, with an absorption rate of about 2.3% over a wide wavelength range, and appears almost transparent. Over the thickness range of several layers of graphene, the absorption rate increases by 2.3% for each additional layer of thickness. Large-area graphene films also have excellent optical properties, and their optical properties change with the change of graphene thickness. This is an unusual low-energy electronic structure for a single layer of graphene. When a voltage is applied to a double-gate bilayer graphene field-effect transistor at room temperature, the graphene's band gap can be adjusted between 0 and 0.25eV. When a magnetic field is applied, the optical response of graphene nanoribbons can be tuned to the terahertz range. |
solubility |
Exhibits good solubility in non-polar solvents and is super hydrophobic and super lipophilic. |
Melting point |
Scientists said in a 2015 study that it was around 4,125K, and there are other studies that suggest the melting point could be around 5,000 K |
Other properties |
Can adsorb and desorb various atoms and molecules. |
Second, chemical properties:
Compounds |
Graphene oxide |
A layered material obtained by graphite oxide. After the bulk phase graphite is treated with a smoky concentrated acid solution, the graphene layer is oxidized into a hydrophilic graphene oxide, and the graphite layer spacing increases from 3.35A before oxidation to 7~ 10A. The separated graphene oxide sheet structure is easily formed by heating or ultrasonic stripping in water. XPS, infrared spectroscopy (IR), solid state nuclear magnetic resonance spectroscopy (NMR) and other characterization results show that graphene oxides contain a large number of oxygen-containing functional groups, including hydroxyl, epoxy functional groups, carbonyl groups, carboxyl groups and so on. The hydroxyl and epoxy functional groups are mainly located on the base surface of graphite, while the carbonyl and carboxyl groups are located on the edge of graphene. |
Graphiane |
Obtained by the reaction of graphene with hydrogen gas, is a saturated hydrocarbon with the molecular formula (CH)n, in which all carbon is sp hybrid and forms a hexagonal network structure, hydrogen atoms are bonded to carbon in alternating forms from both ends of the graphene plane, and graphiane exhibits semiconducting properties with a direct band gap. |
|
Nitrogen-doped graphene or carbon nitride |
After the introduction of nitrogen atoms into the graphene lattice to become nitrogen-doped graphene, the resulting nitrogen-doped graphene shows more excellent properties than pure graphene, in a disordered, transparent, folded gauze shape, some of the sheets are stacked on top of each other to form a multi-layer structure, showing high specific capacitance and good cycle life. |
|
Biocompatibility |
The implantation of carboxyl ions can enable the surface of graphene materials to have active functional groups, thus greatly improving the cellular and biological reactivity of the material. Compared with the tubular form of carbon nanotubes, graphene is more suitable for biomaterial research. And compared with carbon nanotubes, the edges of graphene are longer, easier to be doped and chemically modified, and easier to accept functional groups.
|
|
oxidizability |
Reacts with active metals. |
|
Reducibility |
It can be oxidized in air or by oxidizing acids, by which graphene can be cut into small pieces. Graphene oxide is a layered material obtained by graphite oxidation. It is easy to form separate graphene oxide lamellar structures by heating or ultrasonic stripping in water.
|
|
Addition reaction |
Using the double bonds on graphene, the desired groups can be added through addition reactions.
|
|
Stability |
The structure of graphene is very stable, with a carbon-carbon bond of only 1.42. The bonds between the carbon atoms inside the graphene are flexible, and when an external force is applied to the graphene, the carbon atoms bend and deform, so that the carbon atoms do not have to rearrange to adapt to the external force, which keeps the structure stable. This stable lattice structure gives graphene excellent thermal conductivity. In addition, electrons in graphene do not scatter as they move through their orbits due to lattice defects or the introduction of foreign atoms. Because the interatomic forces are so strong, at room temperature, even when the surrounding carbon atoms collide, the electrons inside graphene are very little disturbed. |
|