- Overview
- Recommended Products
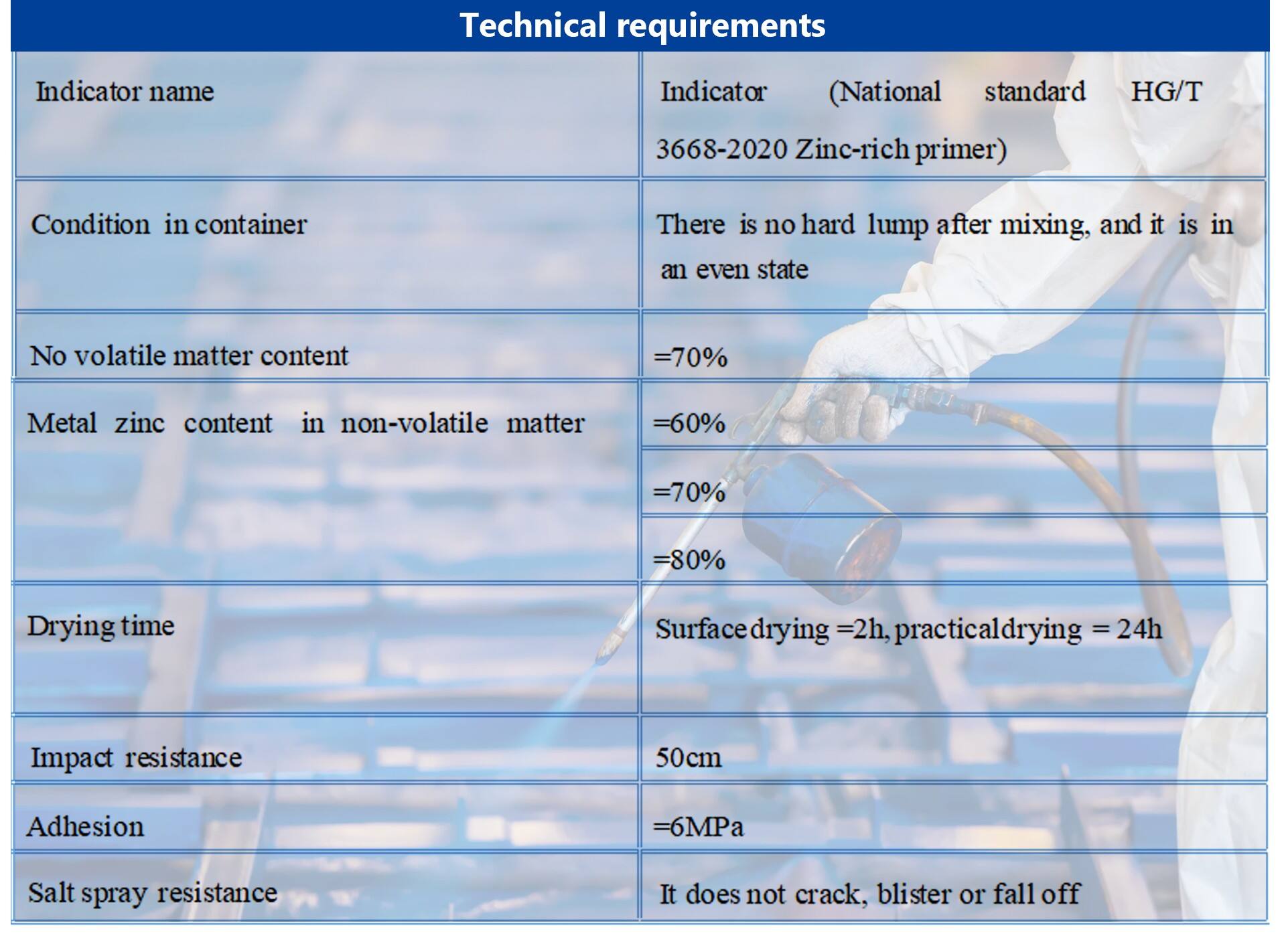
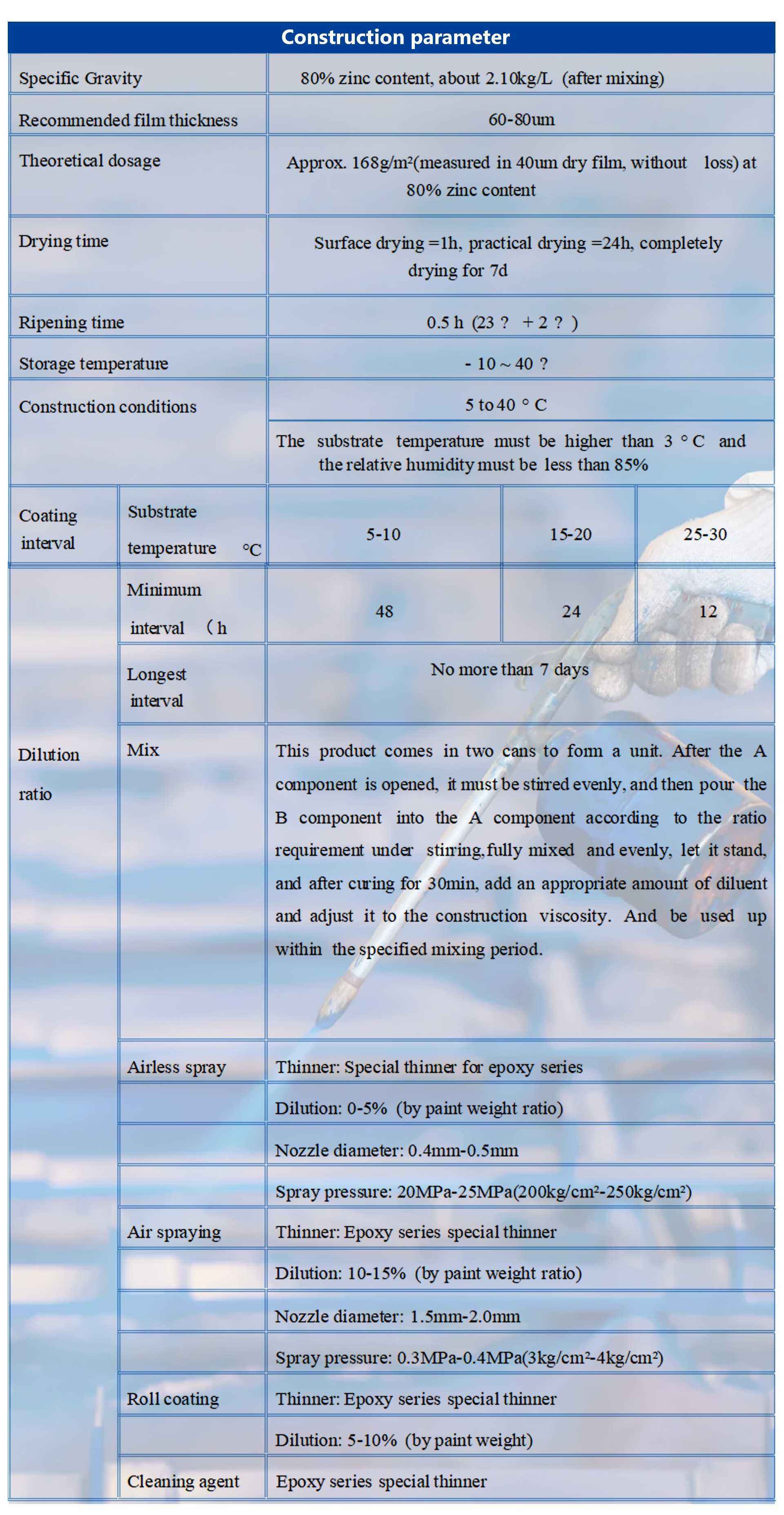
Basic parameters |
※ Color Gray |
※ Proportioning main agent: Curing agent =25:3 |
※ Construction brush coating, spraying, rolling coating can be |
※ The composition is composed of epoxy resin, zinc powder as the main raw materials, |
thickening agent, filler, auxiliary, solvent and so on. |
Product characteristics
Product use
※ It is suitable for the coating of storage tanks, containers, steel structures, steel pipes, offshore platforms, ships, port facilities and harsh anti-corrosion environment, as the bottom coating.
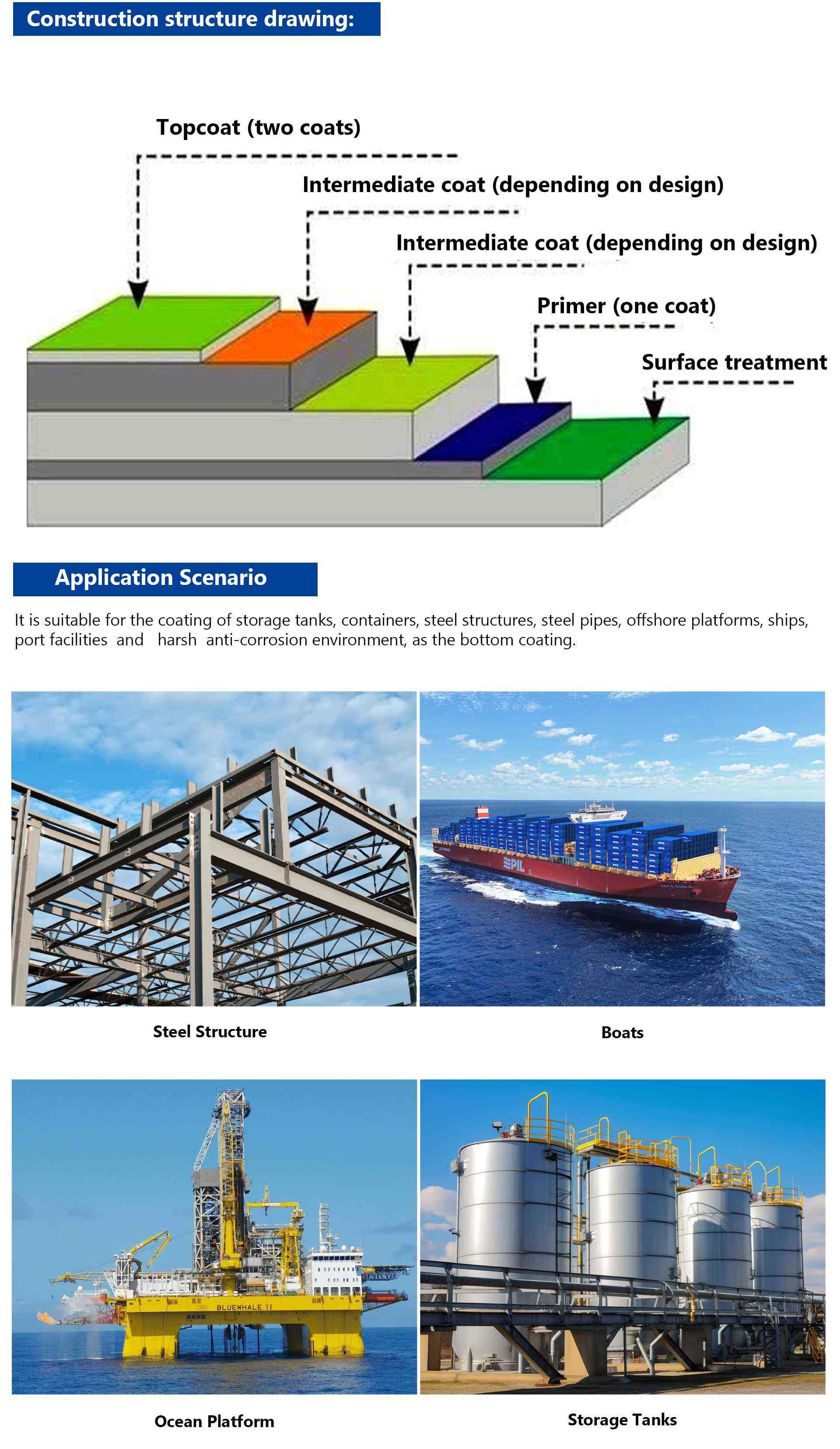
Supporting scheme:
※ Epoxy zinc-rich primer + epoxy cloud iron intermediate paint/epoxy thick paste intermediate paint + acrylic polyurethane finish/polyurethane finish/polysiloxane finish/fluorocarbon finish/epoxy finish/alkyd finish/graphene finish/chlorinated rubber finish, etc
Construction notes:
※ This product is like most zinc-rich paints, long-term exposure to the paint film will appear zinc salt, must be thoroughly cleaned before applying the next paint, otherwise it will affect the adhesion between layers.
※ The temperature of the substrate must be above 3 ° C above the dew point, and when the temperature of the substrate is below 5 ° C, the paint film is not cured and should not be constructed.
※ In the high temperature season construction, easy to occur dry spray, in order to avoid dry spray can be adjusted to dry spray until the diluent.
※ This product should be used by professional painting operators according to the product packaging or the instructions in this manual.
Steel surface:
※ It is necessary to thoroughly remove oil and rust, etc., to achieve the rust removal standard Sa2.5, and the roughness reaches 30um-75um; Adopt manual rust removal method, need to reach the rust removal standard St3 level.
Concrete surface:
※ Concrete surface should be flat, dry, no seepage floating and water. The base that has been polluted by grease and chemicals can be washed with detergent, lye or solvent, and can also be treated by fire baking, steam blowing, etc., but it must not damage the base.
Precautions
※ Products should be stored in a cool and ventilated place to prevent rain, direct
sunlight, avoid collision, need to isolate the fire source.
※ The construction site is strictly prohibited fireworks, painters should wear
glasses, gloves, masks, etc., to avoid skin contact and inhalation of paint mist.
※ All work of coating and use of this product must be carried out in accordance
with various relevant national health, safety and environmental protection regulations and standards.
※ If you have any questions about the use of this product, please contact our technical service department.
Additional:
Principles of cathodic protection
Cathodic protection is an electrochemical protection technology mainly used to prevent corrosion of metal structures in electrolyte environments. The basic principle is to transform the protected metal surface into a cathode by applying an impressed current or using a sacrificial anode, thus inhibiting the corrosion process.
The basic principle of cathodic protection is that by applying an impressed current to the surface of the protected metal structure, it becomes a cathode, so as to inhibit the electron migration of metal corrosion and avoid or weaken the occurrence of corrosion.
Specifically, cathodic protection is achieved through the following steps:
★ Impressed current: By applying an external DC power supply, the metal surface becomes a cathode. This can be done in two ways: the sacrificial anode method and the impressed current method.
★ Sacrificial anode method: An active metal (such as magnesium or aluminum) with a lower electrochemical order than the protected metal is used as the anode, which is connected to the protected metal. These anode materials will preferentially corrode, thereby protecting the protected metal.
★ Impressed current method: Current is applied to the protected metal through an external power source (such as a potentiostat) to make it a cathode. This method is suitable for large structures, such as long-distance pipelines and offshore platforms.
★ Cathode polarization: When the protected metal becomes a cathode, cathode polarization will occur, that is, the potential of the metal is negative shift. This potential change will inhibit the anodizing reaction of the metal, thereby reducing or preventing corrosion.
★ Elimination of electrochemical inhomogeneity: when the potential of the metal is negative to a certain potential value, the electrochemical inhomogeneity of the metal surface is eliminated, and the cathodic dissolution process of corrosion is effectively suppressed to achieve the purpose of protection.